Anacetrapib
CAS No. 875446-37-0
Anacetrapib ( MK 0859 | MK-0859 | MK0859 )
产品货号. M16362 CAS No. 875446-37-0
Anacetrapib (MK 0859) 是一种有效的口服活性胆固醇酯转移蛋白 (CETP) 抑制剂,对 rhCETP 和 C13S CETP 突变体的 IC50 分别为 7.9 nM 和 11.8 nM。
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 2MG | ¥389 | 有现货 |


|
| 5MG | ¥648 | 有现货 |


|
| 10MG | ¥1037 | 有现货 |


|
| 25MG | ¥1887 | 有现货 |


|
| 50MG | ¥3062 | 有现货 |


|
| 100MG | ¥4795 | 有现货 |


|
| 500MG | ¥10287 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称Anacetrapib
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述Anacetrapib (MK 0859) 是一种有效的口服活性胆固醇酯转移蛋白 (CETP) 抑制剂,对 rhCETP 和 C13S CETP 突变体的 IC50 分别为 7.9 nM 和 11.8 nM。
-
产品描述Anacetrapib (MK 0859) is a potent, orally active cholesteryl ester transfer protein (CETP) inhibitor with IC50 of 7.9 nM and 11.8 nM for rhCETP and C13S CETP mutant, respectively; inhibits CETP-mediated cholesterol exchange, resulting in elevated HDL-cholesterol levels and reductions in LDL-cholesterol levels, demonstrates potential to treat elevated cholesterol levels in an effort prevent cardiovascular disease. Atherosclerosis Phase 3 Clinical(In Vitro):Anacetrapib dose-dependently and significantly decreases the transfer of CE from HDL3 to HDL2 (P<0.001 for concentrations equal to and higher than 0.1 μM). Excess Anacetrapib (25 μM) decreases the amount of [14C]Torcetrapib (0.25 μM) binds to immobilized rhCETP by 82% and 60%, respectively. Anacetrapib decreases pre-β-HDL formation by more than 46% (P<0.001) at all concentrations tested (0.1, 1, 3, and 10 μM). A significant reduction of PCSK9 promoter activity by Anacetrapib (ANA) is detected at 3 μM concentration (?22%, p<0.01) and further lowered to 68% of control at 10 μM. Likewise, luciferase activity of B11 cells are decreased by Anacetrapib at 3 μM concentration and reached to a maximal reduction of 38% of control at 10 μM. At 10 μM concentration, Anacetrapib loweres PCSK9 mRNA level to 60% of control and LDLR mRNA level to 67% of control. (In Vivo):Hamsters are given Anacetrapib for 7 days before injection of [3H]cholesterol-labeled macrophages (day 0). Treatment with Anacetrapib leads to significant increases in HDL-C levels at day 0. At day 3, [3H]cholesterol radioactivity in the HDL fraction is significantly increased from control values for Anacetrapib. Anacetrapib (ANA) treatment modestly elevates serum total serum cholesterol levels ~10% (p<0.05) and increases serum LDL-C by 26% (p<0.05) as compared to vehicle control. After an intravenous dose of 0.5 mg/kg, the mean values for systemic plasma clearance, steady-state volume of distribution, and terminal half-life are 2.3 mL/min/kg, 1.1 L/kg, and 12 h, respectively. After oral dosing at 5 mg/kg, the bioavailability of Anacetrapib is 38%. Exposures (AUC) increases in a less than dose-proportional manner from 23 μM?h at 5 mg/kg to 362 μM?h at 500 mg/kg. In this dose range, the peak plasma level (Cmax) ranges from 5 to 26 μM and the time to reach peak plasma level (Tmax) ranged from 3 to 4.5 h.
-
体外实验Anacetrapib dose-dependently and significantly decreases the transfer of CE from HDL3 to HDL2 (P<0.001 for concentrations equal to and higher than 0.1 μM). Excess Anacetrapib (25 μM) decreases the amount of [14C]Torcetrapib (0.25 μM) binds to immobilized rhCETP by 82% and 60%, respectively. Anacetrapib decreases pre-β-HDL formation by more than 46% (P<0.001) at all concentrations tested (0.1, 1, 3, and 10 μM). A significant reduction of PCSK9 promoter activity by Anacetrapib (ANA) is detected at 3 μM concentration (?22%, p<0.01) and further lowered to 68% of control at 10 μM. Likewise, luciferase activity of B11 cells are decreased by Anacetrapib at 3 μM concentration and reached to a maximal reduction of 38% of control at 10 μM. At 10 μM concentration, Anacetrapib loweres PCSK9 mRNA level to 60% of control and LDLR mRNA level to 67% of control.
-
体内实验Hamsters are given Anacetrapib for 7 days before injection of [3H]cholesterol-labeled macrophages (day 0). Treatment with Anacetrapib leads to significant increases in HDL-C levels at day 0. At day 3, [3H]cholesterol radioactivity in the HDL fraction is significantly increased from control values for Anacetrapib. Anacetrapib (ANA) treatment modestly elevates serum total serum cholesterol levels ~10% (p<0.05) and increases serum LDL-C by 26% (p<0.05) as compared to vehicle control. After an intravenous dose of 0.5 mg/kg, the mean values for systemic plasma clearance, steady-state volume of distribution, and terminal half-life are 2.3 mL/min/kg, 1.1 L/kg, and 12 h, respectively. After oral dosing at 5 mg/kg, the bioavailability of Anacetrapib is 38%. Exposures (AUC) increases in a less than dose-proportional manner from 23 μM?h at 5 mg/kg to 362 μM?h at 500 mg/kg. In this dose range, the peak plasma level (Cmax) ranges from 5 to 26 μM and the time to reach peak plasma level (Tmax) ranged from 3 to 4.5 h.
-
同义词MK 0859 | MK-0859 | MK0859
-
通路Metabolic Enzyme/Protease
-
靶点CETP
-
受体MutantCETP(C13S)|rhCETP
-
研究领域Cardiovascular Disease
-
适应症Atherosclerosis
化学信息
-
CAS Number875446-37-0
-
分子量637.5084
-
分子式C30H25F10NO3
-
纯度>98% (HPLC)
-
溶解度10 mM in DMSO
-
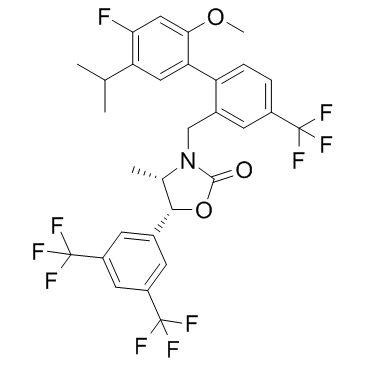
SMILESO=C1O[C@H](C2=CC(C(F)(F)F)=CC(C(F)(F)F)=C2)[C@H](C)N1CC3=CC(C(F)(F)F)=CC=C3C4=CC(C(C)C)=C(F)C=C4OC
-
化学全称2-Oxazolidinone, 5-[3,5-bis(trifluoromethyl)phenyl]-3-[[4'-fluoro-2'-methoxy-5'-(1-methylethyl)-4-(trifluoromethyl)[1,1'-biphenyl]-2-yl]methyl]-4-methyl-, (4S,5R)-
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
1. Krishna R, et al. Lancet. 2007 Dec 8;370(9603):1907-14.
2. Vergeer M, et al. Nat Clin Pract Cardiovasc Med. 2008 Jun;5(6):302-3.
3. Cannon CP, et al. Am Heart J. 2009 Oct;158(4):513-519.e3.
4. Niesor EJ, et al. J Lipid Res. 2010 Dec;51(12):3443-54.
产品手册




关联产品
-
BMS-795311
BMS-795311 是一种有效的口服 CETP 抑制剂,IC50 为 3.8 nM,抑制胆固醇酯 (CE) 转移,IC50 为 0.22 uM。
-
BMS-212122
BMS-212122 (UNII-0Z473OO6GB) is a potent inhibitor of microsomal triglyceride transfer protein (MTP) and has shown hypolipidemic effects in animal studies.BMS-212122 significantly reduced lipid content and monocyte-derived (CD68+) cells in atherosclerotic plaques.
-
CKD-519
CKD-519 (Rocacetrapib, CKD519) 是一种有效的选择性胆固醇酯转移蛋白 (CETP) 抑制剂,抑制人血清中 CETP 介导的胆固醇酯转移,IC50 为 2.3 nM。



 021-51111890
021-51111890 购物车()
购物车()
 sales@molnova.cn
sales@molnova.cn